Residual Bacterial Burden at Closure
This page outlines how intraoperative lavage strategies are evaluated and where Simini Protect Lavage fits within that evidence framework.
- Intraoperative lavage strategies are evaluated by measuring bacterial reduction at the time of closure, the final point under surgical control.
- This approach allows controlled, head-to-head comparison of lavage strategies, independent of infection-rate variability.
- Simini Protect Lavage has been evaluated using these standardized methods as an adjunct to standard saline irrigation.
Representative Evidence: Planktonic Bacterial Reduction
For routine orthopedic procedures, bacteria present at closure are often in a planktonic state rather than a mature biofilm. Standardized in vitro models allow direct comparison of intraoperative lavage solutions under identical exposure conditions.
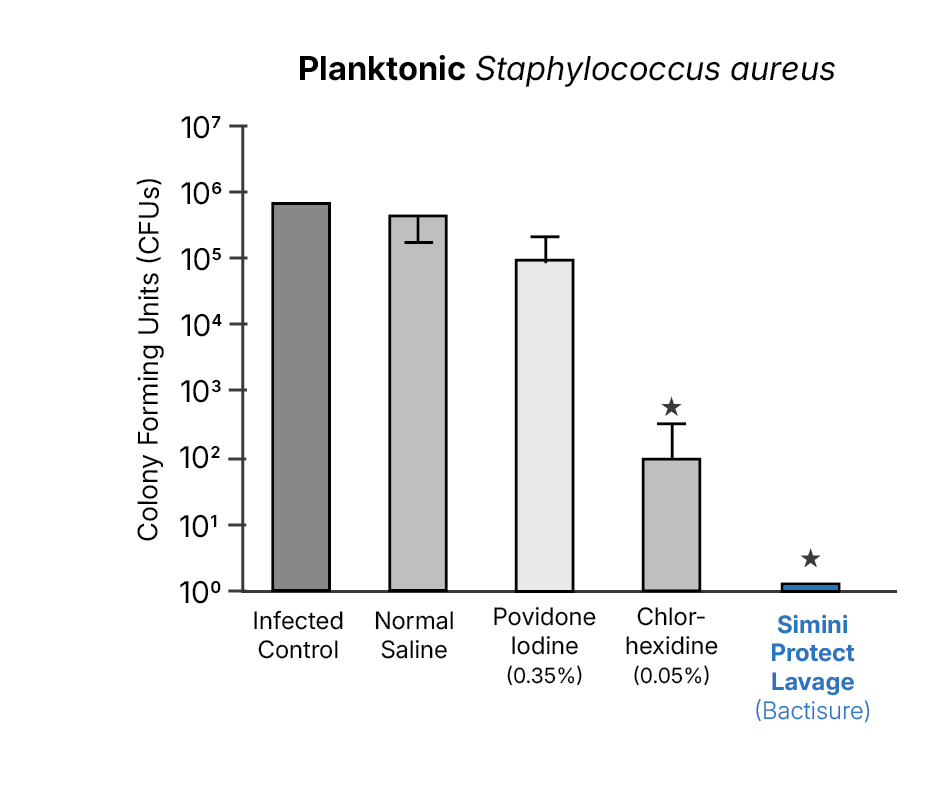
This activity-based evaluation reflects how intraoperative lavage strategies are commonly assessed prior to and independent of infection-rate studies.
Why bacterial burden is measured at closure
Surgical site infection is influenced by multiple perioperative, host-related, and environmental variables. As a result, infection rates alone make it difficult to isolate the effect of individual intraoperative interventions.
For infection-control technologies, investigators have therefore focused on directly measuring residual bacterial burden at the time of closure — the final point under surgical control and the moment at which healing begins.
This approach allows intraoperative interventions to be evaluated using standardized, reproducible endpoints, independent of downstream confounders.
How intraoperative lavage strategies are compared
Bacterial reduction at closure is assessed using controlled, head-to-head microbiologic methods designed to isolate the effect of the lavage itself.
These evaluations typically involve defined bacterial inoculation, standardized lavage protocols, quantitative microbiologic recovery, and direct comparison between lavage strategies under identical conditions.
This framework enables meaningful comparison of intraoperative lavage approaches using measurable, reproducible outcomes, rather than clinical endpoints influenced by multiple external variables.
Evaluation models used in veterinary orthopedic surgery
This evaluation methodology has been applied across multiple validated, standardized models relevant to veterinary orthopedic surgery, including:
- Routine primary procedures assessing removal of planktonic bacteria
- Implant-associated surgery models evaluating bacteria adherent to metallic and porous implant surfaces
- Higher-risk surgical environments, including biofilm-associated conditions and resistant organisms
- In vivo surgical models reflecting intraoperative use under controlled conditions
- Published clinical experience reported in peer-reviewed veterinary orthopedic literature, including case series and observational reports
Together, these models represent the accepted approach for evaluating infection-control technologies used intraoperatively.
Where Simini Protect Lavage fits
Simini Protect Lavage is a non-antibiotic, intraoperative lavage solution designed to be used at closure as an adjunct to standard saline irrigation in veterinary orthopedic surgery.
It has been evaluated using the same standardized models and microbiologic endpoints described above.
Simini is designed to physically remove bacteria, including:
- Planktonic organisms
- Bacteria adherent to implant surfaces
- Biofilm-associated organisms
- Strains with antimicrobial resistance
It integrates easily into standard surgical workflows, without disruption of standard aseptic technique or material change to operative workflow.
Evidence overview
Independent, peer-reviewed evaluations using standardized microbiologic methods have demonstrated greater bacterial removal compared to saline alone across multiple models relevant to veterinary orthopedic surgery.
These evaluations focus on activity-based endpoints at closure, consistent with how other intraoperative infection-control technologies are assessed.
Access the supporting evidence
-
Review the evidence summary Core findings and study data in brief
-
View the evaluation methodology Study design, endpoints, and model overview
-
Access peer-reviewed publications Links to published data