Bacterial Reduction at Surgical Closure
How intraoperative lavage strategies are evaluated
Orientation
This page summarizes key findings from independent studies evaluating bacterial reduction achieved by intraoperative lavage strategies at surgical closure.1-3,12–17
Key findings at a glance
Across independent laboratory, surgical, and clinical evaluations:
- Lower recoverable bacterial burden at closure compared to saline across all evaluated model types3,12–14,17
- Demonstrated activity in implant-associated, biofilm-associated, and resistant organism conditions3,12–14,17
- Lower recoverable bacterial burden than commonly used antiseptic irrigants under standardized comparative conditions3,14,17
- No greater tissue toxicity or wound-healing delay observed compared to saline16
What these studies evaluate
Intraoperative lavage strategies are evaluated by measuring residual bacterial burden at closure, the final point under direct surgical control.6,7
Because surgical site infection rates are influenced by multiple perioperative, host-related, and environmental variables, these studies focus on activity-based endpoints that isolate the effect of the lavage itself under controlled conditions.1–3
This approach allows direct comparison of lavage activity under defined conditions, while recognizing that clinical outcomes are influenced by additional postoperative variables beyond surgical control.
Summary of key findings
Across multiple independent evaluations, Simini Protect Lavage has demonstrated reduced bacterial recovery at closure when assessed using standardized microbiologic methods.3,12-17
These findings are consistent across laboratory models, in vivo surgical models, and published clinical experience in high-risk and revision settings.3,12-17
Key takeaway: Across multiple standardized model types, Simini Protect Lavage demonstrated lower bacterial counts at closure compared to saline under identical conditions, with additional comparative data versus commonly used antiseptic irrigants under standardized conditions.3,12-14,17
Comparison to saline under controlled conditions
Independent laboratory and in vivo surgical models have compared Simini Protect Lavage directly to saline using identical inoculation, lavage, and quantitative recovery protocols.12-14
Across these models, Simini demonstrated reduced bacterial burden at closure under identical conditions.12-14
- Planktonic Staphylococcus aureus (MSSA) in vitro assays14
- Surgical wound models with MRSA and Pseudomonas aeruginosa12
- Murine surgical models reflecting intraoperative conditions12
Comparison to commonly used antiseptic irrigants
In addition to saline, several standardized models have included commonly used antiseptic irrigation solutions—such as dilute povidone iodine (0.35%) and chlorhexidine (0.05%)—to provide comparative context for intraoperative lavage performance.
Using identical inoculation, lavage, and quantitative recovery methods, these studies observed lower recoverable bacterial burden at closure with Simini Protect Lavage compared to these antiseptic solutions under the tested conditions.3,14,17
These comparisons provide additional context for bacterial reduction performance within standardized activity-based evaluation models.
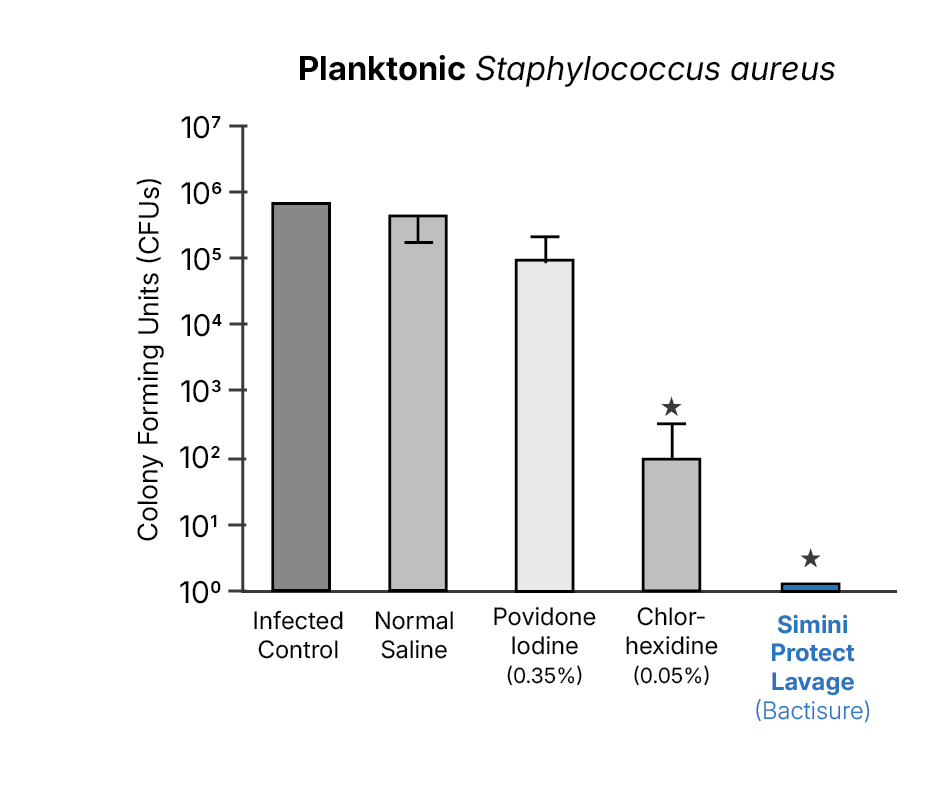
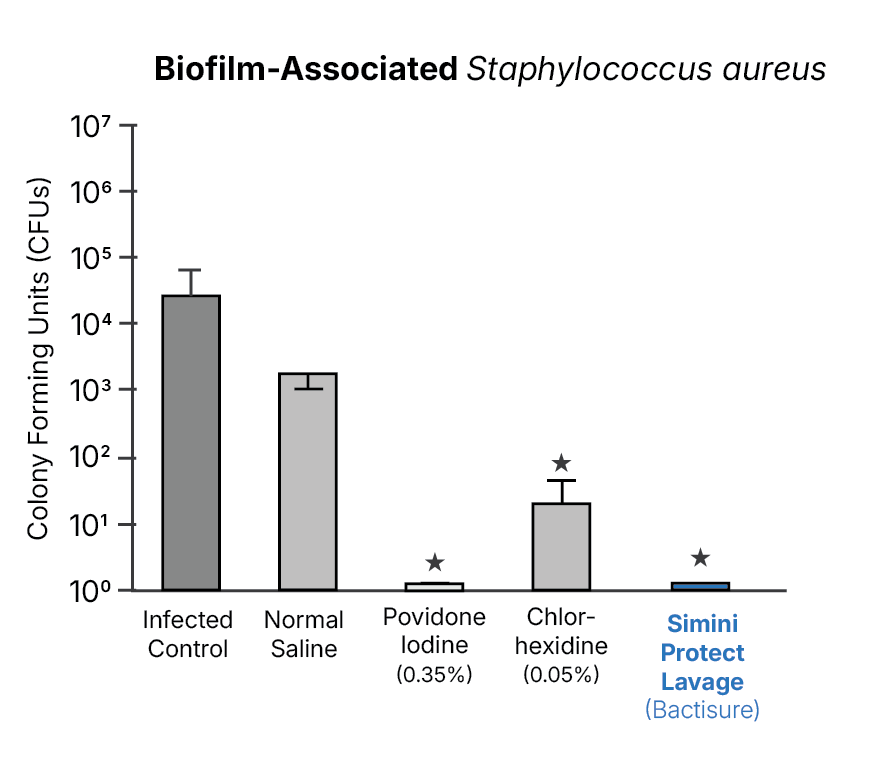
Biofilm-associated and resistant organisms
Biofilm-associated and antimicrobial-resistant organism models show reduced bacterial burden with Simini Protect Lavage under conditions in which saline irrigation alone shows limited effectiveness.14,17
In vivo surgical models
In vivo surgical models designed to reflect veterinary orthopedic procedures demonstrated lower bacterial counts following Simini Protect Lavage compared to saline under standardized conditions.12
Published clinical experience
Veterinary clinical studies and observational reports describe use of Simini Protect Lavage as an adjunct to standard protocols in high-risk and revision orthopedic surgeries, with observations consistent with reduced residual bacterial burden at closure.15
These reports provide real-world context for activity observed in standardized laboratory and surgical models.
Safety and tissue compatibility
Independent safety studies demonstrated no greater cytotoxic effect, no wound healing delay, and compatibility with orthopedic implant materials compared to saline.16
Important context
These studies evaluate bacterial reduction at closure using standardized microbiologic endpoints that isolate intraoperative activity under surgical control.6–8
This approach addresses a different question than infection-rate trials, which reflect multiple postoperative, environmental and host-related variables.
Next steps
References
- Owens CD, Stoessel K. Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect. 2008.
- ASTM ("American Society for Testing and Materials"). Sterilization and disinfection validation standards.
- O’Donnell J, Wu M, Cochrane N, Belay E, Myntti M, James G, Seyler T. Efficacy of common antiseptic solutions against clinically relevant planktonic microorganisms. Orthopedics. 2022.
- Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001.
- Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 2001.
- ASTM International. ASTM E2799-22: Standard test method for evaluating the antimicrobial activity of chemicals and solutions. West Conshohocken, PA; 2022.
- ASTM International. ASTM E3435-24: Standard test method for evaluating antimicrobial efficacy in biofilm or surface-associated models. West Conshohocken, PA; 2024.
- Buckingham-Meyer K, Goeres DM, Hamilton MA. Comparative evaluation of biofilm quantification methods. Biofouling. 2019.
- Campoccia D, Montanaro L, Arciola CR. The significance of infection related to orthopedic devices and implants. Biomaterials. 2006.
- Villegas NA, et al. Surface-associated bacterial persistence and implant-related contamination: model considerations. Small. 2024.
- Costerton JW, et al. Microbial biofilms in nature and disease. Annu Rev Microbiol. 1995.
- Texas Tech University. Murine surgical wound model evaluating intraoperative lavage at closure (MRSA and Pseudomonas aeruginosa). Study summary available in Simini evidence materials.
- Seta J, Pawlitz P, Aboona F, Weaver M, Bou-Akl T, Ren W, Markel D. Efficacy of commercially available irrigation solutions on removal of Staphylococcus aureus and biofilm from porous titanium implants: an in vitro study. J Arthroplasty. 2024;39.
- Hamad C, Sheppard W, Chun R, et al. Comparing the in vitro efficacy of commonly used surgical irrigants for the treatment of implant-associated infections. J Bone Joint Surg Am. 2025.
- Forzisi I, Vezzoni L, Bozzerla M, Vezzoni A. Use of Simini Protect Lavage as an adjuvant in the antiseptic protocol for revision surgeries involving total hip replacement. Vet Comp Orthop Traumatol Open. 2025;8(1).
- Powell A, et al. Effect of commercially available wound irrigation solutions on uninfected host tissue in a murine model. Arthroplasty Today. 2024.
- Kia C, Cusano A, Messina J, et al. Effectiveness of topical adjuvants in reducing biofilm formation on orthopedic implants: an in vitro analysis. J Shoulder Elbow Surg. 2021.
References support the methodology and evidence framework statements summarized on this page.